Part 5: Modern Developments in Protecting Cooling Water Systems
Brad Buecker, Buecker & Associates, LLC
Posted 10/29/2024
Introduction
Part 3 and Part 4 of this series examined important factors that influence scale formation and corrosion in cooling water systems. Control programs for both have been interlinked for decades, but significant changes have occurred, first in the 1980s and now more recently. Per the important theme of employing preventive measures vs. reactive maintenance, we will briefly examine this evolution and highlight modern developments in protecting cooling water systems. Environmental issues related to industrial wastewater discharge are also influencing this evolution. (Read Part 1 and Part 2)
A Simpler Time
Many plants constructed in the last century were located alongside or near freshwater sources. Per the relatively uniform chemistry of freshwaters, by far the most common mineral deposit that can form (in the absence of treatment) in cooling systems is calcium carbonate (CaCO3). However, in accordance with the equilibrium chemistry of carbonate (CO32-), bicarbonate (HCO3–), and carbon dioxide (CO2) species, calcium carbonate does not form in even mildly acidic solutions. So, a very popular treatment program that emerged for open-recirculating (cooling tower-based) systems was sulfuric acid feed for scale control (to establish a pH range of at or near 6.5-7.0), combined with disodium chromate (Na2Cr2O7) for corrosion control. This latter compound provides chromate ions (CrO42-) that react with carbon steel to form a protective pseudo stainless-steel surface layer.
“However, in the 1970s and 1980s dawning recognition of hexavalent chromium (Cr6+) toxicity led to a ban on chromium discharge to the environment, which essentially eliminated chromate treatment for open cooling water systems. The replacement program was quite different, with a key concept being operation at a mildly basic pH (typically around 8.0 or perhaps a bit higher) to assist with corrosion control. The core treatment chemicals became inorganic and organic phosphates. But this more complicated chemistry (as compared to acid-chromate) increased scaling potential.” (1) Figure 1 illustrates the general relationship between corrosion and scaling.

Figure 1. General relationship between corrosion and scaling potential as a function of pH. (1)
When phosphate chemistry emerged as the replacement for acid-chromate, calcium phosphate (Ca3(PO4)2) deposition became very problematic. Formulations evolved that included polyphosphates, organic phosphates (aka phosphonates), polymers, and often a small concentration of zinc; all designed for integrated corrosion and scale control. Corrosion control is not only a function of higher pH but also relies on deposition of reaction products at anodes and cathodes to inhibit ion and electron transfer that drive corrosion reactions. (See Part 4 of this series for a basic description of anodes and cathodes.)
Programs based on phosphate/phosphonate chemistry have been around for decades, but chemistry control is akin to operating on the razor’s edge, where variables including water hardness, pH, water temperature changes, heat exchanger skin temperatures, water flow rates, and other factors can move chemistry out of control ranges. A vivid example of potential problems is shown in the following illustration of a two-pass tube-and-shell heat exchanger, whose cooling water at the time was treated with a traditional phosphate-phosphonate program.


Figures 2a and 2b. Two-pass heat exchanger on a phosphate-phosphonate program just prior to a change in treatment chemistry to a non-phosphorus (non-P) program. (1)
At the inlet end (the lower half of this heat exchanger), corrosion was problematic. At the warmer outlet side (the top half), deposition and scale formation were an issue. So, the former treatment program was not particularly effective for either corrosion or scale control.
Furthermore, discharge of phosphorus-containing compounds has come under increased scrutiny and regulation per the influence of this primary nutrient on algae blooms in receiving bodies of water. As the following photo shows, algae growth can afflict many water bodies, not just those in warm weather climates.
Figure 3. Lake Erie algal bloom, 2017.
Courtesy: https://www.usgs.gov/media/images/lake-erie-algal-bloom

The need for more effective chemistry control coupled with tighter environmental regulations has generated development of alternative chemistry, much of it phosphorus-free.
Back to the Future
For decades, the late world-class cooling water expert, Paul Puckorius, would tell his seminar audiences that the key phrase to always remember with cooling water treatment is, “Protect the metal surface.” Reference 2 outlines pioneering work done in this regard by other experts, who developed non-phosphate chemistry that “interact[s] directly with metal surfaces to form a reactive polyhydroxy starch inhibitor (RPSI) complex that is independent of calcium, pH, or other water chemistry constituents.”
The core compounds establish a stable protective layer, unlike phosphate/phosphonate programs that rely on deposition of reaction products to form the barrier, which, as has been noted, can be difficult to control. Industrial water treatment personnel are increasingly moving from phosphate/phosphonate treatment to non-P chemistry for cooling water corrosion control. But some readers may be wondering about scale control issues in the absence of phosphonates. Effective polymeric compounds have been developed for these needs. The compounds inhibit scale formation through several mechanisms, including sequestration, crystal modification, and crystal dispersion. Figure 4, which illustrates the active sites on many of the most common polymers, will aid in the discussion of these mechanisms.
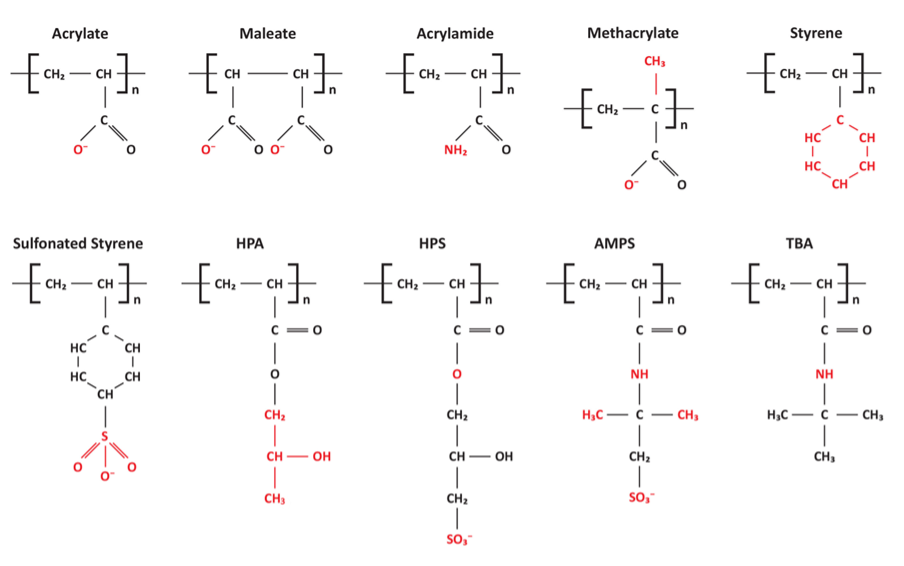
Sequestration
As can be observed in Figure 4, some of the compounds have the same functional groups, i.e., sulfonate (SO3–) and carboxylate (COO–), as those on ion exchange resins for makeup water treatment. For both applications, the negatively charged active sites bind cations, and especially calcium and magnesium. This process sequesters the ions and prevents them from forming scale. Common generic or trade names for these polymers include:
- Polyacrylate (PA)
- Polymethacrylate (PMA)
- Quadsperse
- Flexsperse
Iron presents a challenge to sequestration chemistry, as iron binds strongly to carboxylic and sulfonate sites, reducing their effectiveness.
Crystal Modification
Crystal modification is a prime mechanism for scale control, as illustrated below.


Figures 5a and b. Orderly and distorted growth of calcium carbonate crystals. (1)
Note how the clean crystalline structure in Figure 5a is altered by chemical treatment as shown in 5b. The distorted crystals are fragile and are typically washed away by the flowing water. PA, PMA, and similar compounds are effective for controlling calcium carbonate. For other scale-forming constituents, effective crystal modification may require co- or ter-polymers, which are compounds that have more than one type of reactive moiety.Polymer structure and size have an influence on scale inhibition. Common are molecules of 500–15,000 daltons in size, but in some cases larger polymers may be more effective. Some polymers have been designed to control calcium carbonate, calcium sulfate, and iron-related deposits, and others to control the calcium phosphates that can emerge in phosphate/phosphonate treatment programs, where that chemistry is still being utilized.
Dispersants and Surfactants
Suspended solids in cooling water typically have a negative charge. Many of the polymers outlined in Figure 4 also have a negative charge that enhances repulsion of particles to keep them in suspension.
Fouling of surfaces by oils, greases, or microbiological deposits can inhibit the activity of the corrosion and scale inhibitors outlined above. Of course, root cause control is the ideal method to minimize such fouling, but sometimes other measures are required. Surfactants can assist in breaking down these materials, where cationic, anionic, and nonionic compounds are all available. Many surfactant molecules are similar to detergents in having a polar (water loving) functional group attached to one end of a non-polar organic chain. The organic chains penetrate the oil while the polar functional groups are attracted to water, resulting in dispersion.
Anionic surfactants serve for silt and suspended solids dispersion, while cationic compounds, for example quaternary amines (quats), serve as biodispersants and biocides. In fact, quats are a common material in the fight against macrofouling organisms such as Asiatic clams and zebra mussels. The adult creatures can sense oxidizing biocides and will “clam up” during periods of biocide feed and then re-open when feed stops. The organisms do not detect non-oxidizers and continue to filter water even as the compounds cause lethal damage.
Conclusion
This article highlights some of the most important developments in protecting cooling water systems regarding cooling water scale and corrosion control. There is no “one-size-fits-all” technology. Proper program selection requires coordination and collaboration with experts from a reputable water treatment company. Polymers must be matched with water chemistry to be effective. Some compounds such as dispersants or surfactants, if overfed, may cause excessive foam formation. Changes in water chemistry may require program modifications. A cornerstone for any program is prevention of microbiological fouling. Microbial deposits can defeat the benefits of any of these chemistries.
References
- B. Buecker (Tech. Ed.), “Water Essentials Handbook”; 2023. ChemTreat, Inc., Glen Allen, VA. Currently being released in digital format at www.chemtreat.com.
- Post, R., and Kalakodimi, P., “The Development and Application of Non-Phosphorus Corrosion Inhibitors for Cooling Water Systems”; presented at the World Energy Congress, Atlanta, Georgia, October 2017.

Brad Buecker
Brad Buecker is president of Buecker & Associates, LLC, consulting and technical writing/marketing. Most recently he served as a senior technical publicist with ChemTreat, Inc. He has many years of experience in or supporting the power industry, much of it in steam generation chemistry, water treatment, air quality control, and results engineering positions with City Water, Light & Power (Springfield, IL, USA) and Kansas City Power & Light Company's (now Evergy) La Cygne, KS, USA, station. His work has also included eleven years with two engineering firms, Burns & McDonnell and Kiewit, and he spent two years as acting water/wastewater supervisor at a chemical plant.
Buecker has a B.S. in chemistry from Iowa State University with additional course work in fluid mechanics, energy and materials balances, and advanced inorganic chemistry. He has authored or co-authored over 250 articles for various technical trade magazines, and has written three books on power plant chemistry and air pollution control. He is a member of the ACS, AIChE, AIST, ASME, AWT, the Electric Utility Chemistry Workshop planning committee, and he is active with the International Water Conference and Power-Gen International.
Related Articles
Use P-F Intervals to Map, Avert Failures

The RCM Trap

Can You Really Justify Reliability Centered Maintenance (RCM)?

Design for Maintainability